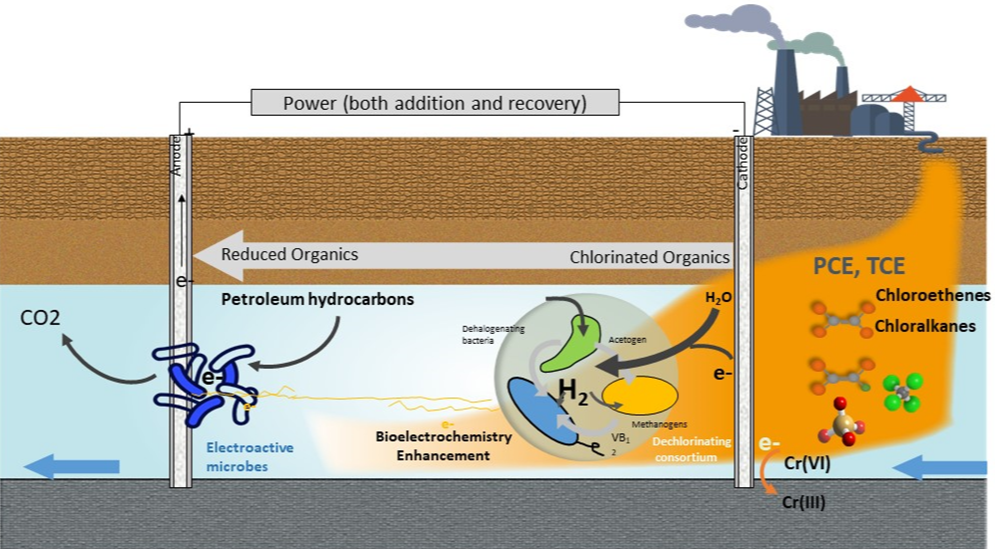
Microbial bioelectrochemical systems are one or two compartment electrochemical devices in which an anode and a cathode favour redox-reactions that are catalysed by microorganisms[1]. The prototype of a bioelectrochemical system is the microbial fuel cell (MFC) that generates electricity based on organic matter oxidation in the anode chamber. Oxygen is used as electron acceptor in the cathode compartment or at the air-cathode. Oxygen in air has effectively unlimited availability and a high standard redox potential. In addition, the bioelectrochemical system can use a small amount of energy to fuel chosen reactions in the cathode chamber. In this case, in the cathode chamber electrons released from the cathode can be exploited for uses other than oxygen reduction. The biocatalytic principle of a bioelectrochemical system contributes to drive thermodynamically unfavourable reactions and can, thus be an alternative electron source for improving PAH removal, for example to achieve significant performance improvements over conventional bioremediation. Â
The technology developed here will benefit from the bioelectrochemical conditions at both the anode and the cathode. At the anode, the anaerobic oxidation of reduced organic compounds (e.g., petroleum hydrocarbons) will be enhanced by the electron acceptor (the anode itself), thus reducing possible sulphate reduction and methane production under anaerobic conditions. At the cathode, reduction reactions will occur such as the dechlorination of chlorinated compounds and the reduction of heavy metals (e.g., CrVI to CrIII). The Chinese research partners’ focus is on chlorinated compounds and heavy metals.
Â
